Calmodulin Definition
All eukaryotic cells contain calcium-modulated protein, or calcium-modulated protein. It acts as a regulator or effector molecule in a wide variety of cellular functions, interacting with other proteins in the cell. Regulation of the cell cycle, intracellular signaling, fertilization, and muscle contraction are among these functions.
Among the calcium-binding proteins involved in muscle contraction is calmodulin, which belongs to the same family as troponin C. Mutations in the genes that encode calmodulin or damage to its binding sites often result in death.
Calmodulin Structure
There are 148 amino acid residues in calmodulin, which is a protein. Several genes encode this protein; in humans, it is encoded by CALM1, CALM2, and CALM3, which are located on chromosomes 14, 2, and 19, respectively. The calmodulin molecule is composed of two globular domains connected by a flexible linker in the middle.
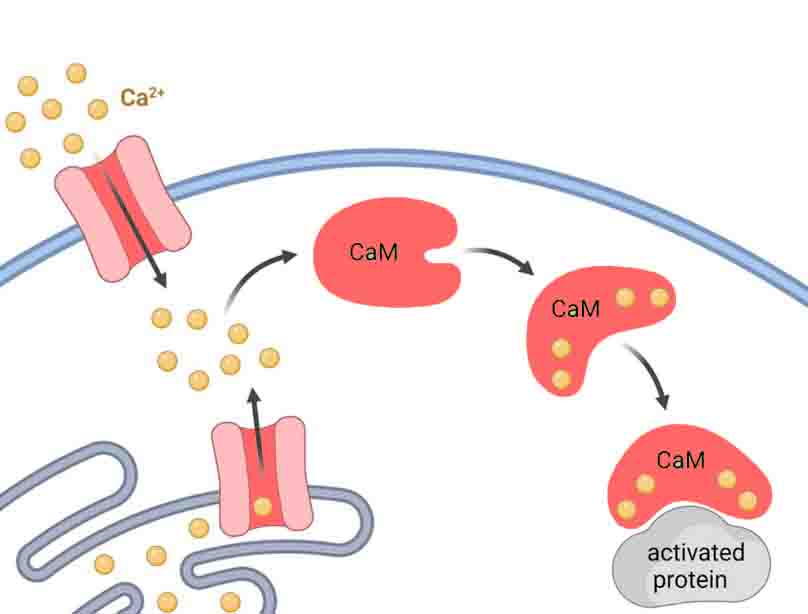
Calmodulin can bind a total of four calcium ions by using motifs called E-F hand motifs, which are ubiquitous in calcium-binding proteins. Aspartate, glutamate, and asparagine are some of the negatively-charged or polar amino acids that make up the calcium binding sites. Amino acids form ionic bonds with Ca2+ ions through their side chains.
Additionally, amino acid residues with oxygen-rich side chains also attract calcium ions. Low Ca2+ concentrations are still able to promote binding with this mechanism.
A conformational change occurs when calcium binds to calmodulin and forms a helix-loop-helix along the backbone. In addition to its conformational changes, calmodulin’s flexible connecting linker allows it to interact with and bind to a wide range of other proteins.
Calmodulin Function
Numerous calcium-mediated processes are regulated by calmodulin. A Ca2+/calmodulin complex is formed when Ca2+ binds to calmodulin, interacting with other proteins. In cellular and physiological processes, these proteins function as enzymes and effectors. In addition to regulating processes indirectly, the Ca2+/calmodulin complex can also directly affect them.
Activating calcium pumps is one of the functions of Ca2+/calmodulin complex. The endoplasmic reticulum or cytoplasmic pumps remove calcium from the cytoplasm. The downstream responses are controlled by controlling calcium levels in the cell.
A Ca2+/calmodulin complex can also function by binding to Ca2+/calmodulin kinases (CAMKs), such as the myosin light chain kinase. As a result of this binding, CAMKs can phosphorylate effector proteins by transferring phosphates from ATP to serine and threonine residues. The proteins then activate downstream processes such as intracellular signaling, smooth muscle contractions, neurotransmitter and hormone synthesis, and cell cycle regulation.
Calcium
The role of calcium in a wide range of physiological processes is becoming increasingly apparent. In the absence of calcium ions, the concentration gradient between the inside and outside of the cell is very large; extracellular calcium concentrations are approximately 1 mM, while calcium ions within the cell are less than 0.1 mM.
Many proteins will readily interact with calcium due to its affinity for interacting with calcium.
Cells receive calcium primarily through gated calcium channels. In addition to being stored in the endoplasmic reticulum, it can also be stored in the kidney. When a specific stimuli is met, calcium channels allow ions to pass through the membrane and into the cell. Depolarization of the membrane or the attachment of a ligand usually causes this to occur.