Hypotonic Solution Definition
A hypotonic solution is a solution that has a lower solute concentration compared to another solution. A solution cannot be hypotonic, isotonic or hypertonic without a solution for comparison. Hypotonic is a description of the solute content of one solution in relation to another solution.
It is used in biology to help scientist describe cells. Knowing the osmolarity (concentration of a solution in number of solutes per liter) of different solutions can show scientists which way the water gradient and solute gradients will form.
Due to the properties of diffusion, every solute in a solution has the tendency to disperse away from each other until evenly distributed. In aqueous solutions, this is caused mainly by the interactions that polar water molecules have on the solutes. The opposite ends of the molecule have different charges, which form temporary bonds, called hydrogen bonds, with other charged portions of solute molecules.
Water molecules cluster around solutes, move them away from the highest concentrations of solutes, and allow more water molecules to move in. Therefore, if you are to pour a hypotonic solution into a hypertonic solution, the solution will initially have areas of high and low concentration but will quickly reach equilibrium.
If these two solutions are separated by a membrane which will only let water through, the water will move out of the hypotonic solution and into the hypertonic solution, until the two are isotonic with each other.
Cells are simply a solution surrounded by a semipermeable bag, the plasma membrane. The plasma membrane is able to keep solutes from diffusing across the cell membrane, while it allows water to diffuse by osmosis across the membrane into the cytoplasm. The membrane is embedded with special proteins, called membrane transport proteins that help transport specific solutes across the membrane.
Other proteins, called aquaporins keep channels open that only water can pass through. All cells must regulate their solute content, to ensure they do not dry up or become too full of water. A cell with a cytosol that is a hypotonic solution to the environment will lose water to the more hypertonic environment that has more solutes. The water, driven to equalize the two solutions, is drawn from the cell.
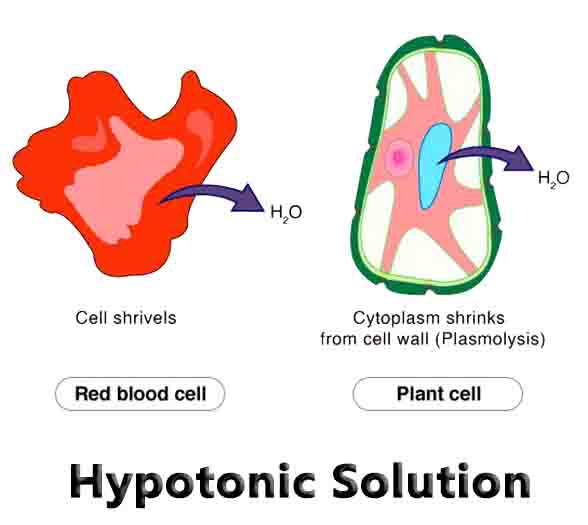
A cell whose cytosol is an extremely hypotonic solution compared to its environment will shrivel up, and is said to be plasmolyzed. This is almost always a bad state for cells, which need water for many chemical reactions.
In the opposite situation, the environment can be a hypotonic solution compared to the cell. In this case, water from the environment tends to diffuse into the cell. If the hypotonic solution of the environment is too strong, the cell may lyse (split open). Cells have many mechanisms for controlling this flow of water. In plant, fungi, and bacterial cells a cell wall is formed around the cell, which keeps it from bursting.
This cell wall is composed of various polysaccharides, proteins, and other molecules. As water fills the cell and pushes against the cell wall, turgor pressure is created. This pressure helps force water back out of the cell, countering the inward flow of water. The image below shows a single plant cell in different environments. The cell on the far right represents a turgid plant cell in a hypotonic solution.
Examples of Hypotonic Solution
Plants and Fungi
Large plants and fungi control the environment around their cells, helping ensure the environment is always a hypotonic solution, compared to the cells. This creates cells that are turgid. The turgid cells push outward on their cell walls, which push against each other creating a rigid structure. The organisms are constantly cycling solutes, to keep the contents of their cells filled with water.
If you’ve ever over-fertilized your garden, you know it is not good for plants. The added solutes in the soil turn the hypotonic solution around the roots into a hypertonic solution. Thus, the roots and entire plant are quickly drained of water. Organisms in this condition will quickly die because they cannot complete the reactions necessary to sustain life.
Animal Cells
Animal cells do not have a cell wall. Typically, animals rely on their skin to separate the outside environment from their inside organs. The fluid inside of their body cavity can then be regulated by a series of membranes and proteins. The fluid will thus remain an isotonic or slightly hypotonic solution in comparison to the cells, keeping them plump and healthy without destroying them.
The process of maintaining the solute concentration in an organism is known as osmoregulation and occurs in all animals. Many animals that live in the ocean have salt glands which expel excess salt from their body. The animals must drink the salt water to get the water into their bodies, but the salts must be concentrated and excreted from the body to maintain it as a hypotonic solution.
Related Biology Terms
- Hypertonic Solution – When a solution has more solutes per liter than another solution.
- Isotonic – When two solutions have the same concentration, and exchange water and solutes at the same rate.
- Osmoregulation – The complex series of cellular mechanisms in organisms which regulates the amount of water and the concentration of each cell’s cytosol.
- Aquaporin – A protein which allows the passage of water through the cell membrane.
FAQ’s
A hypotonic solution is a solution with a lower concentration of solutes compared to another solution. When two solutions with different solute concentrations are separated by a semipermeable membrane, water molecules will move from the area of low solute concentration to the area of high solute concentration, resulting in osmosis.
When a cell is placed in a hypotonic solution, water molecules will move into the cell in an attempt to balance the concentration of solutes between the cell and the solution. This can cause the cell to swell and potentially burst, a process known as lysis. The degree of swelling will depend on the concentration gradient between the cell and the hypotonic solution.
Examples of hypotonic solutions include distilled water and solutions with a low concentration of salt or sugar. In biological systems, hypotonic solutions can also be created by certain medical treatments, such as intravenous fluids.
In medicine, hypotonic solutions can be used to rehydrate the body and increase fluid levels. For example, hypotonic saline solutions may be used to treat dehydration or to dilute medications before administration. However, hypotonic solutions should be used with caution, as they can potentially cause cell lysis in certain situations.