Dendrite Definition
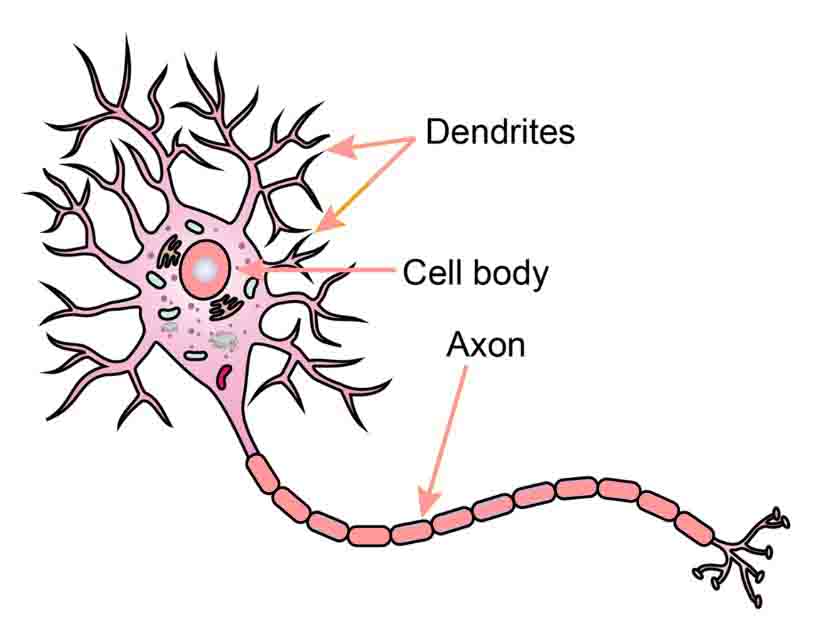
Dendrites are projections of a neuron (nerve cell) that receive signals (information) from other neurons. The transfer of information from one neuron to another is achieved through chemical signals and electric impulses, that is, electrochemical signals.
The information transfer is usually received at the dendrites through chemical signals, then it travels to the cell body (soma), continues along the neuronal axon as electric impulses, and it is finally transferred onto the next neuron at the synapse, which is the place where the two neurons exchange information through chemical signals. At the synapse meet the end of one neuron and the beginning—the dendrites—of the other.
Dendrites Function
The functions of dendrites are to receive signals from other neurons, to process these signals, and to transfer the information to the soma of the neuron.
Receive Information
The dendrites resemble the branches of a tree in the sense that they extend from the soma or body of the neuron and open up into gradually smaller projections. At the end of these projections are the synapses, which is where the information transfer occurs.
More specifically, synapses are the site where two neurons exchange signals: the upstream or pre-synaptic neuron releases neurotransmitters (usually at the end of the neuron, also called axonal terminal) and the downstream or post-synaptic neuron detects them (usually in the dendrites).
At the synapse, the pre-synaptic neuron releases neurotransmitters (number 2 in the figure), which are molecules that the post-synaptic neuron detects. The post-synaptic neuron can detect the neurotransmitters because it has neurotransmitter receptors (number 5 in the figure) to which the neurotransmitters bind.
If the post-synaptic neuron does not have the specific neurotransmitter receptor, then the neurotransmitter will have no effect. Examples of neurotransmitters are dopamine, serotonin, norepinephrine, GABA and glutamate. If, for instance, a pre-synaptic neuron releases dopamine, the post-synaptic neuron will need dopamine receptors in order to detect the signal and consequently receive the information.
Some types of neurons have dendritic spines on the dendrites, which are small protrusions that project from the dendrites and which have neurotransmitter receptors that increase the detection of neurotransmitters.
Process Information
Once the neurotransmitter binds to the neurotransmitter receptor in the post-synaptic neuron, a signaling cascade starts that enables the information to be processed at the synapse. This signaling cascade depends on the neurotransmitter and neurotransmitter receptor: there are excitatory neurotransmitters, such as glutamate, and inhibitory neurotransmitters, such as GABA.
The neurotransmitter receptors begin a signaling cascade that activates certain ligand-gated ion channels. Ligand-gated ion channels enable ions to enter the neuron (e.g. Na+, Ca2+, Cl– or sodium, calcium, chloride, respectively) or to exit the neuron (e.g. K+ or potassium). Let’s take a look at what happens in each case.
In the case of excitatory neurotransmitters, the pre-synaptic neuron releases the neurotransmitter and the post-synaptic neuron detects it when it binds to its specific receptors. Because it is an excitatory neurotransmitter, binding to the receptor will activate ligand-gated ion channels that allow positively charged ions to enter the cell: Na+ and Ca2+. At the same time, some K+ will also exit the cell.
If enough positive charges enter the cell such that the cell membrane potential increases, i.e. there is a net influx of positive charges, then we call this a post-synaptic excitatory potential (EPSP), and the cell is depolarized. If there are enough positive charges such that the cell membrane potential reaches a threshold value, then there is an action potential (see below under Transfer Information).
In the case of inhibitory neurotransmitters, something similar occurs but instead of activating ligand-gated Na+ and Ca2+ channels, binding to the receptor will result in the activation of ligand-gated Cl– channels. Here, Cl– will flow into the post-synaptic neuron. Also, K+ will flow out of the cell. Therefore, a net influx of negative charges (Cl–) lead to a decrease in the cell membrane potential and, consequently, to what we call a post-synaptic inhibitory potential (IPSP). The cell is now hyperpolarized.
Transfer Information
The sum of many EPSPs can surpass the threshold needed for the post-synaptic neuron to start an action potential. To understand this, we need first to understand some intrinsic properties of neurons.
The normal or physiological resting membrane potential of neurons is about -65 mV. This means that the inside of the neuron is negatively charged with respect to the outside of the cell. The reason behind this is that the inside of the cell has some positive charges (K+) and also other negatively charged ions (A–), while the outside of the cell has more positive ions (Na+ and Ca2+) and some negatively charged ones (Cl–). The sum of all charges makes the outside of the cell more positive and the inside of the cell more negative.
When an EPSP occurs in the dendrites, the membrane potential of the post-synaptic neuron increases, for instance from the physiological -65 mV to -64 mV, that is, it becomes less negative.
When the sum of many EPSPs make the membrane potential of the neuron reach a threshold value of about -55 mV, then the neuron fires an action potential that transfers information to the soma and then along the axon to the end of the post-synaptic neuron, reaching at some point the axon terminal, where it will release neurotransmitters onto the next neuron. Action potentials therefore start usually at the dendrites and spread along the neuron.
If the sum of many EPSPs does not reach the threshold needed to start an action potential, then not much happens and the signal is not transferred to the soma or to the axon.
If there are many IPSPs, then more EPSPs are needed to surpass the threshold membrane potential in order to create an action potential.
Dendrites Malfunction
Dendrites play a very important role in information transfer between neurons. It is thus not surprising that malfunctions in dendrites are associated with a variety of disorders of the nervous system.
Malfunctions vary in type and degree of severity, and range from abnormal morphology to disturbances in dendritic branching, anomalies in dendritic development and malfunctioning loss of dendrite branching and dendrite genesis. All of these are linked to disorders such as schizophrenia, autism, depression, anxiety, Alzheimer’s and Down syndrome, among others.
FAQ’s
A dendrite is a branch-like extension of a neuron that receives and processes incoming signals from other neurons. Dendrites are covered in synapses, specialized junctions where incoming signals from other neurons are transmitted to the receiving neuron.
The primary function of dendrites is to receive and integrate signals from other neurons, allowing the neuron to process and respond to incoming information. Dendrites can also modify and amplify incoming signals, and may play a role in the formation of new synapses.
Dendrites and axons are both extensions of neurons, but they differ in their structure and function. While dendrites receive incoming signals, axons transmit outgoing signals to other neurons or to target cells. Additionally, dendrites are typically shorter and more numerous than axons, which can be much longer and often have specialized structures like myelin sheaths.
There are several different types of dendrites, including spiny and aspiny dendrites. Spiny dendrites have small protrusions called spines that can make contact with incoming signals, while aspiny dendrites lack these spines. Additionally, dendrites can differ in their size, shape, and branching patterns, which can impact their function within the neuron.